Lenire Tinnitus Treatment Program
Overview
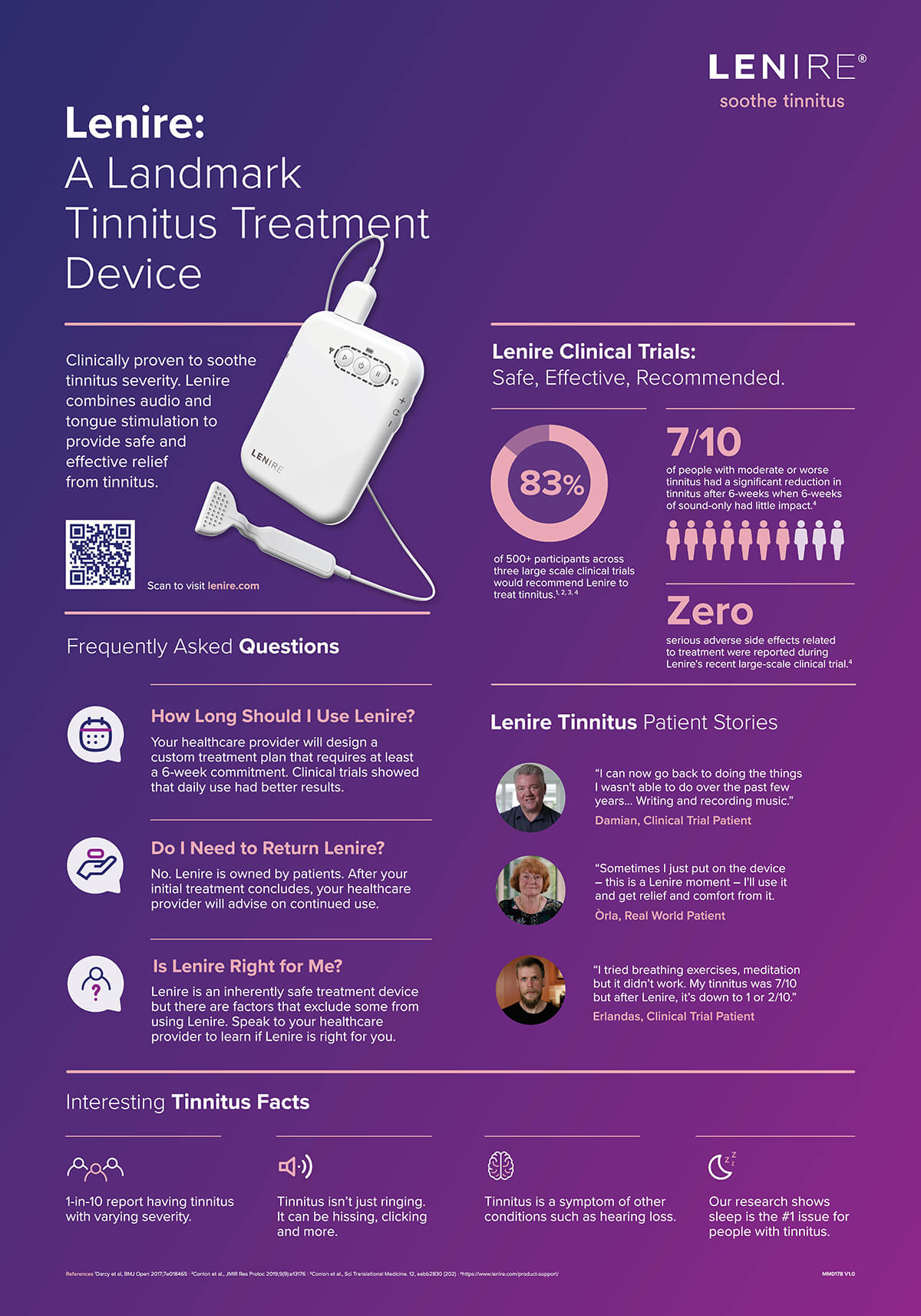
What is Lenire?
Lenire is an FDA-approved medical device designed to treat tinnitus symptoms in a non-invasive and painless way. The lightweight device uses bimodal neuromodulation, a combination of auditory and tongue stimulation, to alter the activity in the brain that contributes to tinnitus. This therapeutic approach utilizes auditory stimulation paired with somatosensory nerve stimulation. Sounds are delivered to the ear while low levels of electric energy are delivered to the tongue. This combination retrains neurons in the brain. Over time this helps the brain learn to reduce its attention and sensitivity to tinnitus subsequently alleviating symptoms.
Components
A light handheld device where you will control your treatment. Begin, pause, or resume your session. Adjust how long your session is. Lower or raise the volume on the headphones or level of tongue stimulation.
These components work together to suppress tinnitus symptoms by targeting two sensory pathways.


How does the Tinnitus Treatment Center of Philadelphia prescribe Lenire?
Tinnitus Consultation
First, you will meet with Dr. Gail Brenner for a HIPAA-compliant telehealth consultation. In this video conference appointment, we start with a structured interview to investigate your medical and hearing health history in order to better understand the specifics of your condition - symptoms, impact, severity, underlying causes, etc. She will also explain the results of your hearing test. Dr. Brenner will make recommendations and determine if you are a candidate for the Lenire Tinnitus Treatment.
*The prerequisites for this appointment are results from a visit with your ENT that includes an audiogram and notes along with completed tinnitus questionnaires.*
Device Fitting
If you are a candidate for the Lenire Treatment Program, we will then schedule your in-person device fitting. Dr. Brenner will calibrate the settings on the Lenire device according to your hearing profile. During your appointment, the tongue tip sensitivity will be calibrated and you will have the opportunity to try the device. Your treatment plan will be created.
Daily Use
Lenire is used 30 minutes – twice a day at home.
Follow-up
You will be scheduled for follow-up appointments with Dr. Brenner to monitor your progress along with a stress reduction program.
At the end of your treatment plan, Dr. Brenner will determine the next steps based on your progress.
Contact Us
Benefits
The development of Lenire marks a new era of tinnitus treatment and has proven to be an effective method of alleviating tinnitus symptoms.
Send a Message
Clinical trials
Over the course of several years the Lenire team has conducted multiple clinical trials. These trials showed that a high percentage of patients experienced long-lasting relief. In the TENT-A3 trial, 204 patients were tested. 79.4 percent of patients experienced clinically significant improvement. In a previous trial, TENT-A1, 326 patients were evaluated over a 12-week period and at the 12-month post-treatment phase. 86.2 percent of patients who completed the 12-week treatment reported improvement. At 12 months post-treatment 83.7 percent found that they experienced long-lasting relief and zero adverse effects.
Easy at home use
Lenire is easy and safe to use. The process of sound and nerve stimulation starts with the press of a button. Administer the treatments from the comfort and quiet of your own home. The Lenire device is yours to keep.

Reclaim
Contact the Tinnitus Treatment Center of Philadelphia today. We can help you find the right solutions to manage your tinnitus and improve your overall health and well-being.